The volatility issue: the monochloramine stability
The science behind the volatility issue
Volatility issue
Chlorine dioxide, being a gas in solution, has the highest volatility.
Hypochlorous acid and monochloramine are nonetheless volatile compounds (Holzvarth 1984).
Hypochlorite, being an ion in solution is not.

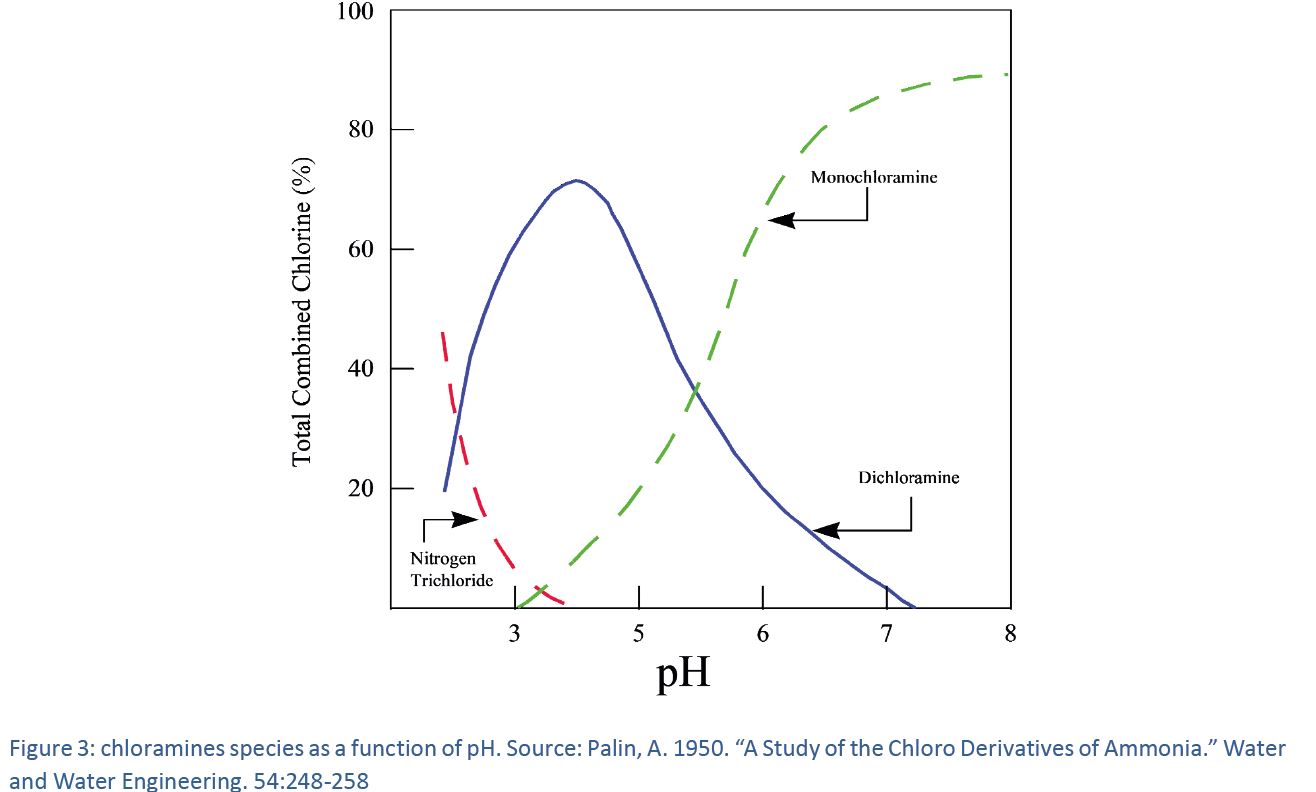
The monochloramine stability
Thus, since hypochlorite and hypochlorous acid are present simultaneously in drinking water and the fraction of hypochlorous acid depends on pH, the volatility of chlorine is much lower at drinking water pH range compared to that of monochloramine.
This of course could be a limitation in open air application where flash off could be an issue but this is not the case for pressurized piped water.
Different situation
In the case of open drinking water storage tanks, the fraction of evaporated monochloramine is normally not an issue (after the first monochloramine molecules evaporates an equilibrium is established and the process stops).
Moreover, being monochloramine is far less aggressive than chlorine, the risk corrosion of wetted and air exposed components is greatly reduced.