Highest stability and SANIKILL monochloramine chemistry
Monochloramine, along with chlorine (sodium hypochlorite) and chlorine dioxide is one of the three EPA listed disinfectant under the Safe Drinking Water Act (SDWA).
Disinfectant stability in water
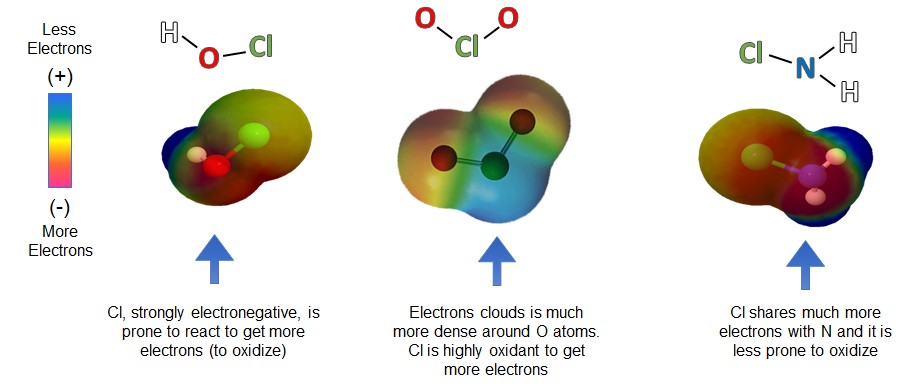
The only difference between monochloramine and the other two disinfectants is the presence of nitrogen (N) instead of oxygen (O) in the molecule. Because the chlorine (Cl) atom in the monochloramine molecule is bonded with nitrogen (N) instead of oxygen, its ability to oxidize is much milder than sodium hypochlorite and chlorine dioxide.
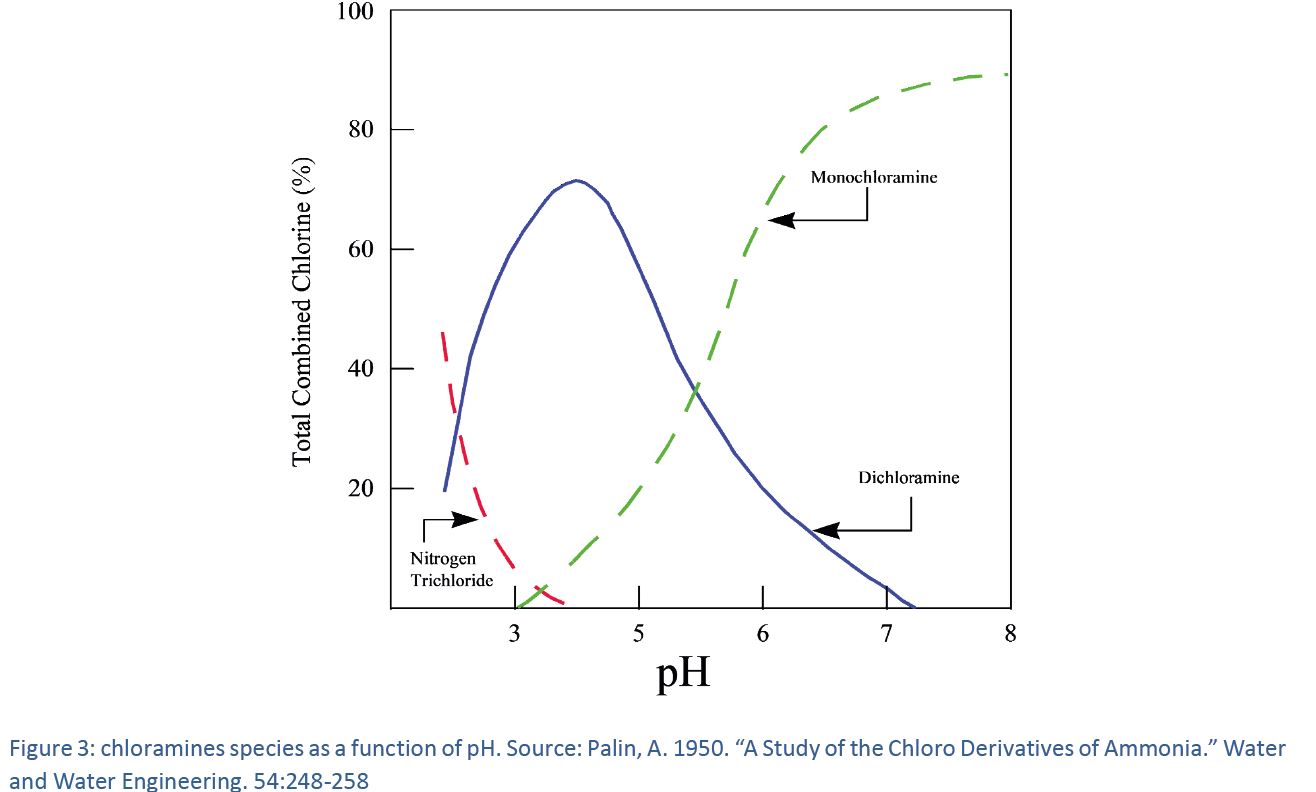
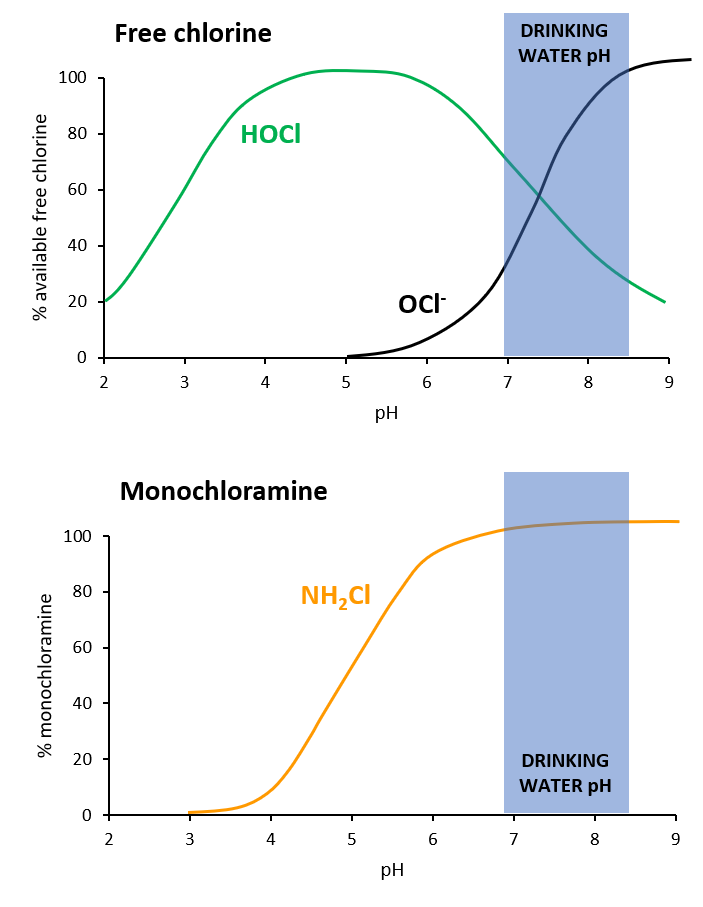
Monochloramine Chemistry
Being a milder oxidizer, the contact time values of monochloramine are higher than that for sodium hypochlorite and chlorine dioxide. This is due to the lower oxidation potential of this disinfectant. This is not a drawback, however, because the stability and low reactivity of monochloramine helps it to maintain a consistent residual throughout the building water system and to better penetrate biofilms. Therefore, monochloramine is the best disinfectant biocide for building supplemental disinfection applications (especially in domestic hot water loops) where long contact time between the disinfectant and the bacteria is achieved.
Biofilm penetration
This feature is very useful to keep out contamination in dead legs during commissioning and renovation. Monochloramine stability helps this biocide remain stable and effective at the proper concentration in low flow regimes (oversized pipes, temporary dead legs, varying flow situations) where other biocides fail.